Cationic palladium(ii)-catalyzed dehydrative nucleophilic substitutions of benzhydryl alcohols with electron-deficient benzenethiols in water†
Abstract
An efficient direct nucleophilic substitution of benzhydryl alcohols with electron-deficient benzenethiols using cationic Pd(II) catalysts as Lewis acids in water is reported. Atom economical and environmentally benign protocols afford S-benzylated products in moderate to excellent yields. Commercially available Pd(MeCN)4(OTf)2, PdCl2(MeCN)2, and Na2PdCl4 are highly efficient catalysts. Notably, this simple protocol can be achieved without any other additives such as acids, bases, or external ligands. A Hammett study on the rate constants of S-benzylation by using various substituted benzhydryl alcohols yielded negative ρ values, suggesting that there is a build-up of positive charge in the transition state.
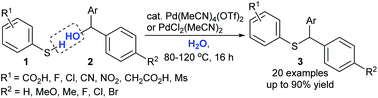

 Please wait while we load your content...
Please wait while we load your content...