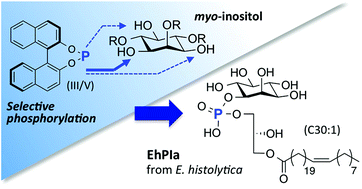
Regioselective phosphorylation of myo-inositol with BINOL-derived phosphoramidites and its application for protozoan lysophosphatidylinositol†
Abstract
A regioselective phosphorylation method for myo-inositol was developed by utilizing readily preparable BINOL-derived phosphoramidites. The method also facilitated the complete separation of the diastereomeric products by simple chromatography. Based on this phosphorylation and Ni-catalyzed alkyl–alkyl cross-coupling reaction for long fatty acids, we achieved the first synthesis of a lysophosphatidylinositol, EhPIa having long fatty acid C30:1, as a partial structure of glycosylphosphatidylinositol (GPI) anchor from the cell membrane of a protozoa, Entamoeba histolytica.

 Please wait while we load your content...
Please wait while we load your content...