Boronic acid/Brønsted acid co-catalyst systems for the synthesis of 2H-chromenes from phenols and α,β-unsaturated carbonyls†
Abstract
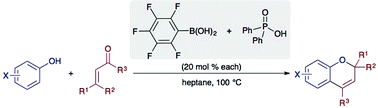
Protocols for the synthesis of substituted 2H-chromenes from α,β-unsaturated carbonyls and phenols are described. Optimal combinations of arylboronic acids and Brønsted acids have been identified, such that both can be employed in catalytic quantities to accelerate these condensations. The method has been used to synthesize a variety of substituted 2H-chromenes, as well as photochromic naphthopyrans. The use of pentafluorophenylboronic acid and diphenylphosphinic acid enabled an expansion of the electrophile scope to include α,β-unsaturated ketones. Hall's ‘phase-switching’ of boronic acids has been exploited to achieve the separation of the two co-catalysts from unpurified reaction mixtures by a simple liquid–liquid extraction.

 Please wait while we load your content...
Please wait while we load your content...