pH-Dependent membrane lysis by using melittin-inspired designed peptides†
Abstract
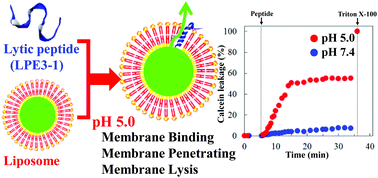
We developed a membrane-lytic peptide (LP) having 26 amino acid residues composed of a helix-promoting hydrophobic segment (Leu–Ala repetitive sequence) and a cationic segment from melittin. In the presence of liposomes, LP interacts with liposomal surfaces to form a hydrophobic helix in the lipid bilayer in a wide pH range. In order to provide LP with a weakly acidic (endosomal) pH-controlled membrane-lytic activity, we have designed an LPE peptide series (a typical peptide, LPE3-1) with a hydrophobic segment in which Leu (L) residues are replaced by acidic Glu (E) residues. To analyze the pH-selective membrane-lytic activity of the designed peptides, both calcein leakage and membrane accessibility assays were performed. In the case of membrane disruption induced by the active pore formation, the incorporated calcein would leak from the liposomes and simultaneously the aqueous solution in the membrane surrounding would be accessible to the liposome interior at pH 5.0. The assays in the presence of LPE3-1 indicated no significant leakage or accessibility at pH 7.4, but a typical leakage and some accessibility to liposomes were positively observed at pH 5.0. In order to estimate whether the weakly acidic pH-controlled lytic activity is due to a secondary structural change of the hydrophobic segment of LPE3-1 in the liposome membrane, we have measured circular dichroism spectra. In the presence of liposomes, the minimum showing the characteristic helical structure was observed at 222 nm only under weakly acidic conditions. This pH dependence is in good agreement with the results from the leakage and accessibility assays. The pH-dependent membrane disruption properties of LPE3-1 may open a new avenue to gain insight into the interaction between peptides and lipids for the development of efficient drug/gene delivery systems.
 Please wait while we load your content...
Please wait while we load your content...