Effects of heterocyclic-based head group modifications on the structure–activity relationship of tocopherol-based lipids for non-viral gene delivery†
Abstract
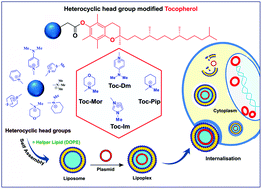
Gene therapy, a promising strategy for the delivery of therapeutic nucleic acids, is greatly dependent on the development of efficient vectors. In this study, we designed and synthesized several tocopherol-based lipids varying in the head group region. Here, we present the structure–activity relationship of stable aqueous suspensions of lipids that were synthetically prepared and formulated with 1,2-dioleoyl phosphatidyl ethanolamine (DOPE) as the co-lipid. The physicochemical properties such as the hydrodynamic size, zeta potential, stability and morphology of these formulations were investigated. Interaction with plasmid DNA was clearly demonstrated through gel binding and EtBr displacement assays. Further, the transfection potential was examined in mouse neuroblastoma Neuro-2a, hepatocarcinoma HepG2, human embryonic kidney and Chinese hamster ovarian cell lines, all of different origins. Cell-uptake assays with N-methylpiperidinium, N-methylmorpholinium, N-methylimidazolium and N,N-dimethylaminopyridinium head group containing formulations evidently depicted efficient cell uptake as observed by particulate cytoplasmic fluorescence. Trafficking of lipoplexes using an endocytic marker and rhodamine-labeled phospholipid DHPE indicated that the lipoplexes were not sequestered in the lysosomes. Importantly, lipoplexes were non-toxic and mediated good transfection efficiency as analyzed by β-Gal and GFP reporter gene expression assays which established the superior activity of lipids whose structures correlate strongly with the transfection efficiency.

 Please wait while we load your content...
Please wait while we load your content...