Structure–property relationships of photoresponsive inhibitors of the kinesin motor†
Abstract
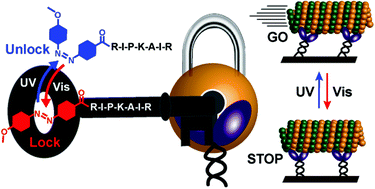
Recently we demonstrated the photoregulation of the activity of kinesin-1 using an azobenzene-tethered peptide (azo-peptide: Azo-Ile-Pro-Lys-Ala-Ile-Gln-Ala-Ser-His-Gly-Arg-OH). To understand the mechanism behind this photoswitchable inhibition, here we studied the structure–property relationships of a range of azo-peptides through systematic variations in the structures of the peptide and azobenzene units. The vital peptide sequence for kinesin inhibition—mediated through electrostatic, hydrophobic and C–H⋯π interactions—was the same as that for the self-inhibition of kinesin. We also identified substituents on the azobenzene capable of enhancing the photoswitchability of inhibition. As a result, we developed a new inhibitor featuring a relatively short peptide unit (-Arg-Ile-Pro-Lys-Ala-Ile-Arg-OH) and an azobenzene unit bearing a para-OMe group. In the trans form of its azobenzene unit, this finely tuned inhibitor stopped the kinesin-driven gliding motility of microtubules completely at a relatively low concentration, yet allowed gliding motility with a relatively high velocity in the cis form obtained after UV irradiation.

 Please wait while we load your content...
Please wait while we load your content...