Selenocysteine containing analogues of Atx1-based peptides protect cells from copper ion toxicity†
Abstract
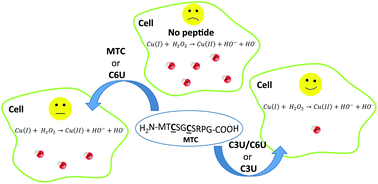
Seleno-substituted model peptides of copper metallochaperone proteins were analyzed for the metal affinity and in vitro anti-oxidative reactivity. An acyclic MTCXXC (X is any amino acid) reference peptide previously analyzed as a potent inhibitor of ROS production underwent substitution of the cysteine residues with selenocysteine to give two singly substituted derivatives C3U and C6U and the doubly substituted analogue C3U/C6U. Presumably due to the softer nature of Se vs. S, all selenocysteine containing peptides demonstrated high affinity to Cu(I), higher than that of the reference peptide, and in the same order of magnitude as that measured for the native protein, Atox1. A stronger impact of residue 3 confirmed previous findings on its more dominant role in metal coordination. In vitro studies on the HT-29 human colon cancer cell line, MEF mice embryonic fibroblasts, and MEF with the knocked-out Atox1 gene (Atox1 −/−) consistently identified C3U/C6U as the most potent inhibitor of ROS cellular production based on the 2′,7′-dichlorodihydrofluorescin diacetate (H2DCF-DA) assay, also in comparison with known drugs employed in the clinic for Wilson's disease. The selenocysteine containing peptides are thus promising drug candidates for chelation therapy of Wilson's disease and related conditions relevant to excessive copper levels.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...