An efficient approach to construct benzisothiazol-3(2H)-ones via copper-catalyzed consecutive reaction of 2-halobenzamides and carbon disulfide†
Abstract
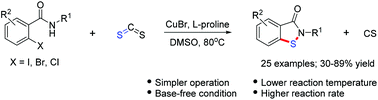
An efficient copper-catalyzed reaction for the synthesis of benzisothiazol-3(2H)-ones has been developed, starting from easily available 2-halobenzamides and carbon disulfide, which gave the corresponding target products in 30–89% yield for 25 examples. The reaction proceeds via a consecutive process with S–C bond and S–N bond formation.
 Please wait while we load your content...
Please wait while we load your content...