Chemoenzymatic synthesis and pH-responsive properties of amphoteric block polysaccharides†
Abstract
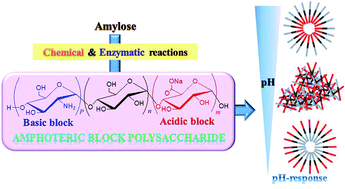
Here, we investigated the chemoenzymatic synthesis of α(1→4)-linked amphoteric block polysaccharides. Amylouronic acid as an acidic block was first synthesized by 2,2,6,6-tetramethylpiperidine 1-oxyl-mediated oxidation of a water-soluble amylose (chemical reaction). A short maltooligosaccharide chain, serving as an initiating site for the following enzymatic polymerization, was then introduced at the nonreducing end of the product by thermostable α-glucan phosphorylase-catalyzed enzymatic oligomerization of α-D-glucose 1-phosphate. Finally, thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of α-D-glucosamine 1-phosphate from the produced primer provided a basic block at the nonreducing end, leading to the desired amphoteric block polysaccharides. The structures of the products at each step were determined by 1H NMR analysis. Furthermore, amphoteric products exhibited specific inherent isoelectric points (pIs). When the pH-responsive properties in aqueous solutions were evaluated using a divalent acid and base, similar hierarchical assembling/disassembling processes were observed by shifting the pH values from the pI to both the acidic and basic pH.

 Please wait while we load your content...
Please wait while we load your content...