Design, synthesis and in vitro splicing inhibition of desmethyl and carba-derivatives of herboxidiene†
Abstract
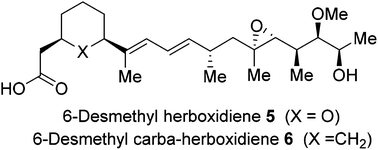
Herboxidiene is a potent inhibitor of spliceosomes. It exhibits excellent anticancer activity against multiple human cancer cell lines. Herein, we describe an enantioselective synthesis of a desmethyl derivative and the corresponding carba-derivatives of herboxidiene. The synthesis involved Suzuki coupling of a vinyl iodide with boronate as the key reaction. For the synthesis of carba-derivatives, the corresponding optically active cyclohexane-1,3-dicarbonyl derivatives were synthesized using an enantioselective desymmetrization of meso-anhydride. The biological properties of these derivatives were evaluated in an in vitro splicing assay.

 Please wait while we load your content...
Please wait while we load your content...