Visible-light-mediated, nitrogen-centered radical amination of tertiary alkyl halides under metal-free conditions to form α-tertiary amines†
Abstract
A mild and operationally convenient amino-functionalization of a range of tertiary alkyl halides by reaction with iminoiodinanes (PhI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNs) and I2 has been developed. According to the mechanistic experiments described within, the reaction is speculated to proceed through a light-promoted, N-centered radical pathway involving a N,N-diiodosulfonamide reactive species. This method of direct N-incorporation offers an attractive alternative to the production of α-tertiary amines, a synthetically challenging structural class found in a variety of bioactive molecules.
NNs) and I2 has been developed. According to the mechanistic experiments described within, the reaction is speculated to proceed through a light-promoted, N-centered radical pathway involving a N,N-diiodosulfonamide reactive species. This method of direct N-incorporation offers an attractive alternative to the production of α-tertiary amines, a synthetically challenging structural class found in a variety of bioactive molecules.
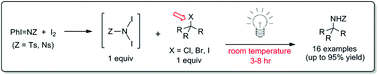

 Please wait while we load your content...
Please wait while we load your content...