Regioselective oxidation of unprotected 1,4 linked glucans†
Abstract
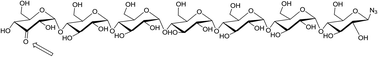
Palladium-catalyzed alcohol oxidation allows the chemo- and regioselective modification of unprotected 1,4 linked glucans. This is demonstrated in the two-step bisfunctionalization of 1,4 linked glucans up to the 7-mer. Introduction of an anomeric azide is followed by a highly regioselective mono-oxidation of the terminal C3–OH functionality. The resulting orthogonal bis-functionalized oligosaccharides are a viable alternative to PEG-spacers as demonstrated in the conjugation of a cysteine mutant of 4-oxalocrotonate tautomerase with biotin.

 Please wait while we load your content...
Please wait while we load your content...