Palladium-catalyzed direct desulfitative C2 arylations of 3-halo-N-protected indoles using (hetero)arenesulfonyl chlorides†
Abstract
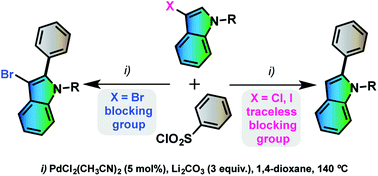
The direct arylation of N-protected 3-haloindole derivatives with benzenesulfonyl chlorides as coupling partners using 5 mol% of bis(acetonitrile)dichloropalladium(II) catalyst and lithium carbonate as a base in 1,4-dioxane was investigated. We demonstrated that both iodo and chloro substituents at the indolyl C3 position act as temporary blocking groups allowing the formation of 2-arylindoles through a direct desulfitative arylation, followed by in situ dehalogenation. While, from 3-bromoindole derivatives, 2-aryl-3-bromoindoles were obtained without debromination, and could be converted into 2,3-diarylindoles through a second palladium coupling. This method allows one to prepare in a few steps a very wide variety of indole derivatives, which are of interest in the synthesis of bioactive molecules.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery
 Please wait while we load your content...
Please wait while we load your content...