Two phenyls are better than one or three: synthesis and application of terminal olefin-oxazoline (TOlefOx) ligands†
Abstract
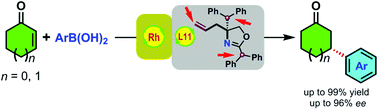
A novel terminal olefin-oxazoline ligand was introduced into rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to enones and gave excellent enantioselectivities. The two phenyls proved better than one or three in ligand evaluations.

 Please wait while we load your content...
Please wait while we load your content...