Divergent copper-mediated dimerization and hydroxylation of benzamides involving C–H bond functionalization†
Abstract
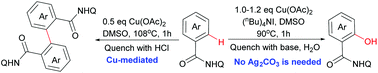
Convenient methods were developed for copper-mediated oxidative C–H activation of aminoquinoline benzamides. The reaction conditions can be tuned to give either hydroxylation or dimerization compounds as the major products efficiently. Preliminary mechanistic studies suggested that different coordination states of copper may lead to different reaction outcomes.
 Please wait while we load your content...
Please wait while we load your content...