A biocatalytic approach to capuramycin analogues by exploiting a substrate permissive N-transacylase CapW†
Abstract
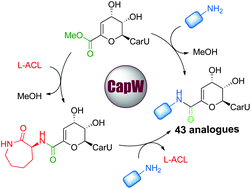
Using the ATP-independent transacylase CapW required for the biosynthesis of capuramycin-type antibiotics, we developed a biocatalytic approach for the synthesis of 43 analogues via a one-step aminolysis reaction from a methyl ester precursor as an acyl donor and various nonnative amines as acyl acceptors. Further examination of the donor substrate scope for CapW revealed that this enzyme can also catalyze a direct transamidation reaction using the major capuramycin congener as a semisynthetic precursor. Biological activity tests revealed that a few of the new capuramycin analogues have significantly improved antibiotic activity against Mycobacterium smegmatis MC2 155 and Mycobacterium tuberculosis H37Rv. Furthermore, most of the analogues are able to be covalently modified by the phosphotransferase CapP/Cpr17 involved in self resistance, providing critical insight for future studies regarding clinical development of the capuramycin antimycobacterial antibiotics.


 Please wait while we load your content...
Please wait while we load your content...