Synthesis of chiral lactams via asymmetric hydrogenation of α,β-unsaturated nitriles†
Abstract
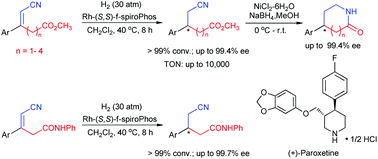
A highly efficient Rh-catalyzed enantioselective hydrogenation of α,β-unsaturated nitriles containing ester/amide groups has been developed. Under mild conditions, with a complex of rhodium and (S,S)-f-spiroPhos as the catalyst, a variety of α,β-unsaturated nitriles bearing an ester or amide group were successfully hydrogenated to the corresponding chiral nitriles with excellent enantioselectivities (up to 99.7% ee) and high turnover numbers (TON = 10 000). Furthermore, this catalyst system was also successfully applied to the synthesis of important chiral pharmacophore fragments, lactams, Paroxetine and amino acids.

 Please wait while we load your content...
Please wait while we load your content...