Modifying the phenyl group of PUGNAc: reactivity tuning to deliver selective inhibitors for N-acetyl-d-glucosaminidases†‡
Abstract
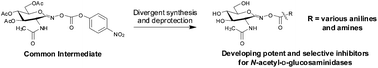
The synthesis of analogues of the potent N-acetylhexosaminidase inhibitor, PUGNAc, are described. These compounds were assayed against a set of biologically important N-acetyl-D-glucosaminidases and were found to vary in both potency and selectivity.

 Please wait while we load your content...
Please wait while we load your content...