The stereoselectivities of tributyltin hydride-mediated reductions of 5-bromo-d-glucuronides to l-iduronides are dependent on the anomeric substituent: syntheses and DFT calculations†
Abstract
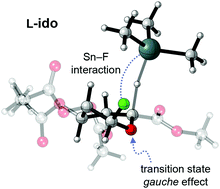
One of the shortest synthetic routes to L-iduronic acid derivatives is via free radical reduction of the C-5 bromide of the corresponding protected D-glucuronic acid derivative. The epimerization of such C-5 bromides to the L-ido derivatives via reaction with tributyltin hydride was investigated. It was found that the stereoselectivity of the reaction was dependent on the anomeric substituent. If the substituent was fluoride the L-ido product was obtained exclusively in 65–72% yield whereas the O-methyl or O-acetyl derivatives led to isomeric mixtures of both the L-ido and D-gluco products in different ratios depending on the reaction conditions. DFT calculations were performed to determine the stereoelectronic factors that favour formation of the L-ido isomer from the fluoride and suggest the selectivity is due to a transition state gauche effect and an Sn–F interaction.


 Please wait while we load your content...
Please wait while we load your content...