Transition-metal free thiocyanooxygenation of functionalized alkenes: facile routes to SCN-containing dihydrofurans and lactones†
Abstract
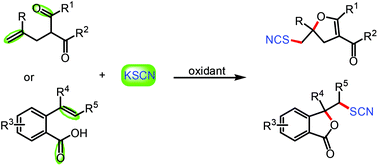
An efficient transition-metal free tandem cyclization of functionalized alkenes with easily available thiocyanate salts has been developed under mild conditions. This protocol offers a simple, easy-to-handle, and atom-economical method for the synthesis of SCN-containing dihydrofurans and lactones with good to excellent yields. A detailed mechanistic investigation indicates that a tandem radical pathway is involved in this transformation.

 Please wait while we load your content...
Please wait while we load your content...