Rhodium-catalyzed ortho C–H bond activation of arylamines for the synthesis of quinoline carboxylates†
Abstract
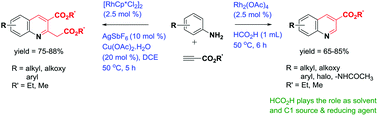
The rhodium catalyzed annulation of anilines with alkynic esters allowing for the high-yield synthesis of quinoline carboxylates with excellent regioselectivity is described. This unprecedented reaction employs either formic acid as the C1 source and reductant or copper(II) as the oxidant and is proposed to proceed via rhodacycle of in situ generated amide and enamine ester followed by ortho C–H activation of arylamines with rhodium as the catalyst.

 Please wait while we load your content...
Please wait while we load your content...