Comparison of boron-assisted oxime and hydrazone formations leads to the discovery of a fluorogenic variant†
Abstract
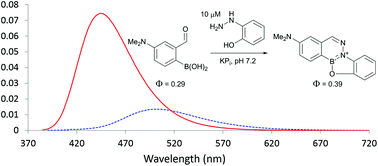
We use kinetic data, photophysical properties, and mechanistic analyses to compare recently developed high-rate constant oxime and hydrazone formations. We show that when Schiff base formation between aldehydes and arylhydrazines is carried out with an appropriately positioned boron atom, then aromatic B–N heterocycles form irreversibly. These consist of an extended aromatic structure amenable to the tailoring of specific properties such as reaction rate and fluorescence. The reactions work best in neutral aqueous buffer and can be designed to be fluorogenic – properties which are particularly interesting in bioconjugation.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...