Deracemizing organocatalyzed Michael addition reactions of 2-(arylthio)cyclobutanones with β-nitrostyrenes†‡
Abstract
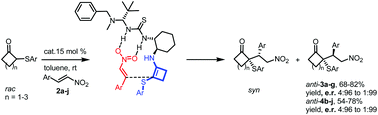
Organocatalyzed Michael addition reactions of 2-(arylthio)cyclobutanones with trans-β-nitrostyrenes have been carried out using a bifunctional thiourea-primary amine catalyst, providing diastereoisomerically and enantiomerically enriched 2-alkyl-2-(arylthio)cyclobutanones having two contiguous stereocenters of which one is a chiral quaternary center. The absolute configuration of these novel adducts was assigned by X-ray diffraction analysis and a transition-state model is proposed to explain the observed stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...