The first catalytic asymmetric cycloadditions of imines with an enolisable anhydride†
Abstract
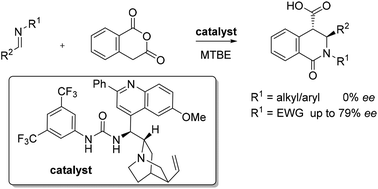
The first catalytic, asymmetric reactions of imines with homophthalic anhydride to form disubstituted 3,4-dihydroisoquinolones are reported. The use of N-mesyl aldimines is key, as more basic imines undergo rapid uncatalysed reactions, while imines possessing larger N-sulphonyl substituents form lactams with lower ee.

 Please wait while we load your content...
Please wait while we load your content...