Concise asymmetric syntheses of novel phenanthroquinolizidines†
Abstract
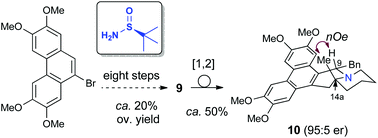
The first preparation of enantioenriched phenanthroquinolizidines with a quaternary center at C14a was accomplished in seven steps from readily available starting materials. Key steps were an efficient dynamic kinetic allylation of a diastereomeric mixture of chiral tert-butylsulfinyl ketimines and the construction of a piperidine E ring by rhodium catalyzed hydroformylation. The Stevens rearrangement of the corresponding N-benzyl derivatives took place smoothly, allowing the installation of a benzyl moiety at C9 in a trans relationship with the methyl group. The cytoxicity of the prepared phenanthroquinolizidines was evaluated against different human cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...