Oxidative ring-opening of ferrocenylcyclopropylamines to N-ferrocenylmethyl β-hydroxyamides†
Abstract
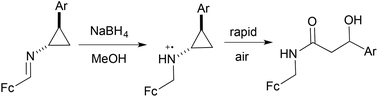
The in situ reduction of ferrocenyl cyclopropylimines to the corresponding amines triggers a facile oxidative ring-opening to yield the formal four-electron oxidation products: N-ferrocenylmethyl β-hydroxyamides. This process is believed to proceed via generation of a ferrocinium ion in the presence of air, leading to facile formation of a distonic radical cation that is ultimately trapped by oxygen.

 Please wait while we load your content...
Please wait while we load your content...