Radical-mediated dehydrogenation of bile acids by means of hydrogen atom transfer to triplet carbonyls†
Abstract
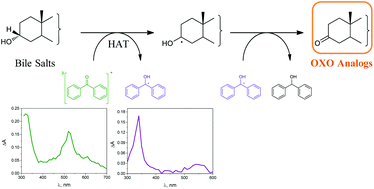
The aim of the present paper is to explore the potential of radical-mediated dehydrogenation of bile salts (BSs), which is reminiscent of the enzymatic action of hydroxysteroid dehydrogenase enzymes (HSDH). The concept has been demonstrated using triplet carbonyls that can be efficiently generated upon selective UVA-excitation. Hydrogen atom transfer (HAT) from BSs to triplet benzophenone (BP) derivatives gave rise to radicals, ultimately leading to reduction of the BP chromophore with concomitant formation of the oxo-analogs of the corresponding BSs. The direct reactivity of triplet BP with BSs in the initial step was evaluated by determining the kinetic rate constants using laser flash photolysis (LFP). The BP triplet decay was monitored (λmax = 520 nm) upon addition of increasing BS concentrations, and the obtained rate constant values indicated a reactivity of the methine hydrogen atoms in the order of C-3 < C-12 < C-7. The steady-state kinetics of the overall process, monitored through the disappearance of the typical BP absorption band at 260 nm, was much faster under N2 than under O2, also supporting the role of the oxygen-quenchable triplet in the dehydrogenation process. Furthermore, irradiation of deaerated aqueous solutions of sodium cholate in the presence of KPMe provided the oxo-analogs, 3[O],7[O]-CA, 3[O]-CA and 7[O]-CA, arising from the HAT process.

 Please wait while we load your content...
Please wait while we load your content...