Synthesis, structure and pyrolysis of stabilised phosphonium ylides containing saturated oxygen heterocycles†
Abstract
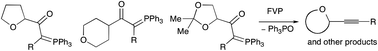
A range of twelve stabilised phosphonium ylides containing tetrahydrofuran, tetrahydropyran or 2,2-dimethyl-1,3-dioxolane rings have been prepared and fully characterised, including one X-ray structure determination of each type. The X-ray structures confirm the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functions to be syn and all the compounds undergo thermal extrusion of Ph3PO to give the corresponding alkynes. In some cases there is also competing loss of Ph3P to give different carbene-derived products and evidence has been obtained for the generation of 2-phenyloxete in this way. Raising the pyrolysis temperature leads in several cases to new secondary reactions of the alkyne products involving a sequence of alkyne to vinylidene isomerisation, intramolecular CH insertion, and retro Diels Alder reaction.
O functions to be syn and all the compounds undergo thermal extrusion of Ph3PO to give the corresponding alkynes. In some cases there is also competing loss of Ph3P to give different carbene-derived products and evidence has been obtained for the generation of 2-phenyloxete in this way. Raising the pyrolysis temperature leads in several cases to new secondary reactions of the alkyne products involving a sequence of alkyne to vinylidene isomerisation, intramolecular CH insertion, and retro Diels Alder reaction.

 Please wait while we load your content...
Please wait while we load your content...