Gold-catalyzed three-component spirocyclization: a one-pot approach to functionalized pyrazolidines†
Abstract
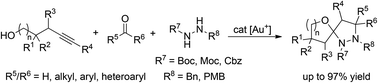
An efficient, highly atom economic synthesis of hitherto unknown spirocyclic pyrazolidines in a one-pot process was developed. The gold-catalyzed three-component coupling of alkynols, hydrazines and aldehydes or ketones likely proceeds via cycloisomerization of the alkynol to an exocyclic enol ether and subsequent [3 + 2]-cycloaddition of an azomethine ylide. A library of 29 derivatives with a wide range of functional groups was synthesized in up to 97% yield. With this new method, every position in the final product can be substituted which renders the method ideal for applications in combinatorial or medicinal chemistry.

 Please wait while we load your content...
Please wait while we load your content...