A versatile synthesis of chiral β-aminophosphines†
Abstract
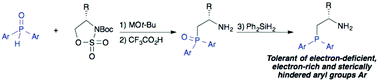
A method for the preparation of chiral β-aminophosphines having substituted P-aryl groups is described. Ring-opening of cyclic sulfamidates with metal diarylphosphinites yields β-aminophosphine oxides, which are then reduced to the corresponding phosphines. Effects of the diarylphosphinite countercation on the regioselectivity of the ring-opening reaction (P- versus O-alkylation) are discussed. This method enables the introduction of electron-deficient, electron-rich and sterically hindered diarylphosphino groups, as demonstrated by the synthesis of a series of novel, P-aryl-substituted β-aminophosphines derived from tert-leucinol, valinol and phenylglycinol. Access to these derivatives will create new opportunities for steric and electronic tuning of β-aminophosphine-derived chiral ligands and organocatalysts.
- This article is part of the themed collection: New Talent

 Please wait while we load your content...
Please wait while we load your content...