Chemoenzymatic synthesis of enantiopure hydroxy sulfoxides derived from substituted arenes†
Abstract
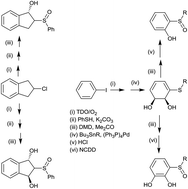
Enantiopure β-hydroxy sulfoxides and catechol sulfoxides were obtained, by chemoenzymatic synthesis, involving dioxygenase-catalysed benzylic hydroxylation or arene cis-dihydroxylation and cis-diol dehydrogenase-catalysed dehydrogenation. Absolute configurations of chiral hydroxy sulfoxides were determined by X-ray crystallography, ECD spectroscopy and stereochemical correlation. The application of a new range of β-hydroxy sulfoxides as chiral ligands was examined.

 Please wait while we load your content...
Please wait while we load your content...