Enantioselective palladium-catalyzed arylation of N-tosylarylimines with arylboronic acids using a chiral 2,2′-bipyridine ligand†
Abstract
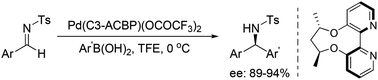
With the aid of an axially chiral 2,2′-bipyridine ligand, we have successfully developed a palladium-catalyzed method for the enantioselective arylation of N-tosylarylimines, furnishing the chiral diarylmethamines with high yields and enantioselectivities under very mild conditions. An exogenous base was avoided and imine hydrolysis was inhibited in this transformation.

 Please wait while we load your content...
Please wait while we load your content...