Copper-free Sandmeyer cyanation of arenediazonium o-benzenedisulfonimides†
Abstract
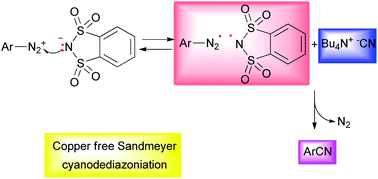
Arene and heteroarenediazonium o-benzenedisulfonimides can be used as efficient reagents in Sandmeyer cyanation. This work reports such reactions carried out by us under very mild conditions using tetrabutyl ammonium cyanide as a safe cyanide source and, interestingly, without the need for a Cu catalyst. The reactions have given rise to aryl nitriles in good yields (25 examples, average yield 75%). A good amount of o-benzenedisulfonimide was recovered from each reaction and then reused to prepare other salts. Mechanistic insights have allowed us to highlight the fundamental role of the o-benzenedisulfonimide anion as an electron transfer agent.

 Please wait while we load your content...
Please wait while we load your content...