One-pot preparation of trifluoromethylated homoallylic N-acylhydrazines or α-methylene-γ-lactams from acylhydrazines, trifluoroacetaldehyde methyl hemiacetal, allyl bromide and tin†
Abstract
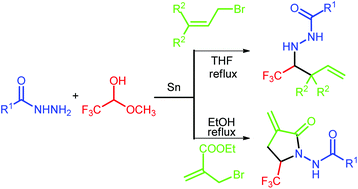
An efficient and convenient one-pot method for the preparation of trifluoromethylated homoallylic N-acylhydrazines or α-methylene-γ-lactams has been described. In this process, allyl bromide and metal tin are used instead of toxic stannanes, and commercially available aqueous trifluoroacetaldehyde methyl hemiacetal was used as a trifluoromethyl source.

 Please wait while we load your content...
Please wait while we load your content...