Syntheses and cellular investigations of di-aspartate and aspartate-lysine chlorin e6 conjugates†
Abstract
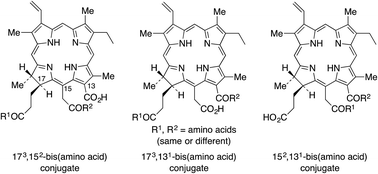
Chlorin e6 is a tricarboxylic acid degradation product of chlorophyll a. Four chlorin e6 bis(amino acid) conjugates were regioselectively synthesized bearing two aspartate conjugates in the 131,173- and 152,173-positions, or at the 131,152via an ethylene diamine linker. One additional conjugate bearing two different amino acids, lysine at 131via an ethylene diamine linker and an aspartate at 152via a β-alanine linker was also synthesized. The cytotoxicity and uptake of four di(amino acid) chlorin e6 conjugates were investigated in human HEp2 cells, and compared with chlorin e6. The most cytotoxic and most taken up conjugates were the zwitterionic 131,152-disubstituted conjugates 28 and 33; these also localized in multiple organelles. In contrast, the anionic 131,173- and 152,173-di-aspartyl chlorin e6 conjugates 12 and 13 showed low dark cytoxicity and lower phototoxicity compared with chlorin e6.

 Please wait while we load your content...
Please wait while we load your content...