Facile synthesis of aza-spirocyclopropanyl oxindoles by the reaction of 3-(2-bromoethyl)-indole with 2,3-dimethylimidazole-1-sulfonyl azide triflate†
Abstract
3-(2-Bromoethyl)indole reacts with 2,3-dimethylimidazole-1-sulfonyl azide triflate to give an intermediate N-(2,3-dimethylimidazole)-1-sulfonyl aza-spirocyclopropanyloxindole. This reactive species is captured by an alcohol or amine to afford the corresponding aza-spirooxindole sulfonate and sulfonamide.
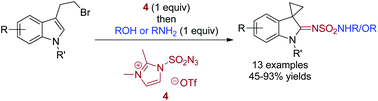

 Please wait while we load your content...
Please wait while we load your content...