Design, synthesis and antiproliferative activity of the new conjugates of E7010 and resveratrol as tubulin polymerization inhibitors†
Abstract
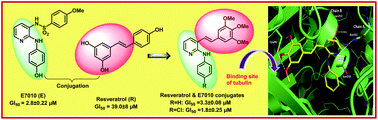
A new class of (E)-N-phenyl-3-styrylpyridin-2-amine conjugates were designed and synthesized on the basis of E7010 and resveratrol scaffolds. These conjugates were evaluated for their antiproliferative activity in four human cancer cell lines with GI50 values ranging from 2.1 μM to 20 μM. Two of the conjugates RSV-1 and RSV-11 were found to possess 13-fold higher GI50 values than resveratrol and 1 to 2 fold higher GI50 values than E7010 against the human cervical HepG2 cancer line. They displayed high potency and selectivity in a panel of NCI 60 human cancer cell lines. Based on the GI50 values against the panel of 60 NCI cancer cell lines and dock scores from the molecular modelling studies, we selected RSV-1 and RSV-11 for tubulin polymerization and mechanistic studies. Furthermore, RSV-1 and RSV-11 compounds inhibited the assembly of tubulin by strongly binding to the colchicine-binding site. The G2/M-phase is arrested in HepG2 cells as assessed by flow cytometry. Structure based studies, western blotting and immunofluorescence experiments demonstrated that RSV-1 and RSV-11 depolymerize microtubules in the HepG2 cell line, resulting in an accumulation of G2/M cells.

 Please wait while we load your content...
Please wait while we load your content...