One-pot synthesis of carbazoles via tandem C–C cross-coupling and reductive amination†
Abstract
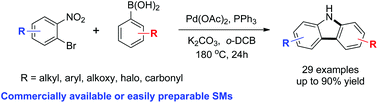
We have developed a highly efficient synthetic route to carbazoles that employs sequential C–C/C–N bond formation via Suzuki cross-coupling and Cadogan cyclization using commercially available or easily preparable starting materials. The developed method is compatible with electron neutral, rich or deficient substrates. The synthetic utility of this method was demonstrated by the concise syntheses of four natural products (glycozoline, glycozolicine, glycozolidine and clausenalene).

 Please wait while we load your content...
Please wait while we load your content...