Synthesis of α-haloenamides via zinc halide mediated direct addition of benzhydryl halides to ynamides†
Abstract
A regio- and stereoselective synthesis of highly substituted α-haloenamides was described via the zinc halide mediated direct addition of benzhydryl halides to ynamides under mild conditions. The products α-haloenamides were further transformed into multisubstituted enamides via Suzuki and Sonogashira cross-coupling reactions.
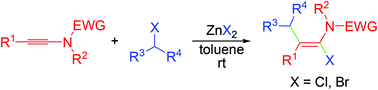

 Please wait while we load your content...
Please wait while we load your content...