Synthesis of amino heterocycle aspartyl protease inhibitors
Abstract
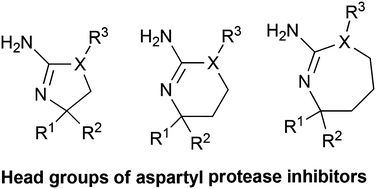
Aspartyl proteases are important pharmacological targets. Historically aspartyl proteases have been commonly targeted with transition state derived peptidomimetics. The strategy to develop aspartyl protease inhibitors has undertaken a dramatic paradigm shift in the last 10 years. The pharmaceutical industry in 2005 disclosed several scaffolds or “head groups” that prompted the field to move beyond peptidomimetic derived inhibitors. Since the discovery of the first amino heterocycle aspartyl protease inhibitor, the amino hydantoin, industry and academia have positioned themselves for a foothold on the new molecular space, designing a variety of related “head groups”. Both the design and synthetic efforts involved in constructing these scaffolds are varied and complex. Here we highlight the synthetic strategies used to access these amino heterocycle scaffolds.


 Please wait while we load your content...
Please wait while we load your content...