Efficient electrochemical water oxidation in neutral and near-neutral systems with a nanoscale silver-oxide catalyst†
Abstract
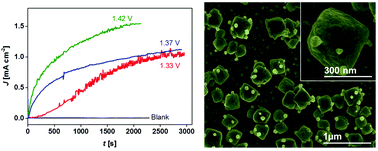
In electrocatalytic water splitting systems pursuing for renewable energy using sunlight, developing robust, stable and easily accessible materials operating under mild chemical conditions is pivotal. We present here a unique nanoparticulate type silver-oxide (AgOx-NP) based robust and highly stable electrocatalyst for efficient water oxidation. The AgOx-NP is generated in situ in a HCO3−/CO2 system under benign conditions. Micrographs show that they exhibit a nanoscale box type squared nano-bipyramidal configuration. The oxygen generation is initiated at low overpotential, and a sustained O2 evolution current density of >1.1 mA cm−2 is achieved during prolonged-period water electrolysis. The AgOx-NP electrocatalyst performs exceptionally well in metal-ion free neutral or near-neutral carbonate, phosphate and borate buffers relative to recently reported Co-oxide and Ni-oxide based heterogeneous electrocatalysts, which are unstable in metal-ion free electrolytes and tend to deactivate with time and lose catalytic performance during long-term experimental tests.


 Please wait while we load your content...
Please wait while we load your content...