Valence and oxide impurities in MoS2 and WS2 dramatically change their electrocatalytic activity towards proton reduction†
Abstract
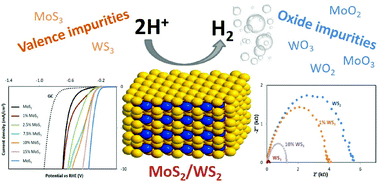
Layered molybdenum disulfide (MoS2) and tungsten disulfide (WS2) have received renewed interest in recent years as they are catalytic towards the hydrogen evolution reaction (HER) and they are touted as future replacements of platinum in electrolyzers. There is a significant discrepancy in the found onset potentials of MoS2 and WS2 towards the hydrogen evolution reaction. Here we show that the presence of valence sulfide impurities, such as MoS3 and WS3, and their oxide counterparts, such as MoO2, MoO3 and WO2, WO3 can contribute to the catalytic activity towards hydronium reduction to hydrogen of MoS2 and WS2. Therefore, it is highly possible that the differences in the reported onset potentials and thus catalytic activities of the MoS2 and WS2 are due to the presence of catalytic impurities.

 Please wait while we load your content...
Please wait while we load your content...