SnO2 as a high-efficiency polysulfide trap in lithium–sulfur batteries†
Abstract
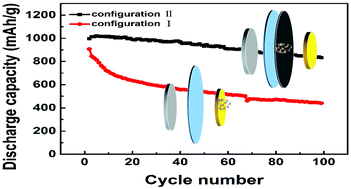
The ithium–sulfur battery stands as one of the most promising successors of traditional lithium-ion batteries due to its super high theoretical energy density, but practical application still suffers from the shuttle effect arising from soluble intermediate polysulfides. Here, we report SnO2 as a chemical adsorbent for polysulfides. As an interlayer between the cathode and separator, SnO2 gives better results to prevent the polysulfides from diffusing to the lithium anode than as a modifier of the carbon matrix directly. The lithium–sulfur battery with an SnO2 interlayer delivers an initial reversible capacity of 996 mA h g−1 and retains 832 mA h g−1 at the 100th discharge at 0.5 C, with a fading rate of only 0.19% per cycle. The improvements benefit from the quasi-open space provided by the interlayer configuration for the diffused sulfur species, which can largely relieve the loss of active substances caused by the volume effect during the lithiation/delithiation process.

 Please wait while we load your content...
Please wait while we load your content...