Force measurements reveal how small binders perturb the dissociation mechanisms of DNA duplex sequences†
Abstract
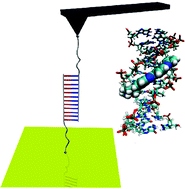
The force-driven separation of double-stranded DNA is crucial to the accomplishment of cellular processes like genome transactions. Ligands binding to short DNA sequences can have a local stabilizing or destabilizing effect and thus severely affect these processes. Although the design of ligands that bind to specific sequences is a field of intense research with promising biomedical applications, so far, their effect on the force-induced strand separation has remained elusive. Here, by means of AFM-based single molecule force spectroscopy, we show the co-existence of two different mechanisms for the separation of a short DNA duplex and demonstrate how they are perturbed by small binders. With the support of Molecular Dynamics simulations, we evidence that above a critical pulling rate one of the dissociation pathways becomes dominant, with a dramatic effect on the rupture forces. Around the critical threshold, we observe a drop of the most probable rupture forces for ligand-stabilized duplexes. Our results offer a deep understanding of how a stable DNA–ligand complex behaves under force-driven strand separation.

 Please wait while we load your content...
Please wait while we load your content...