Sintering-resistant Pt@CeO2 nanoparticles for high-temperature oxidation catalysis†
Abstract
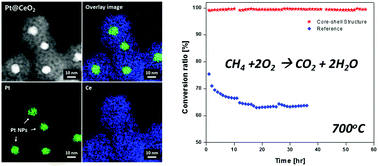
The key challenge that has limited the industrial utilization of nano-sized metal catalysts is their poor thermal stability and the resulting performance degradation. Here, we address this issue by designing a post-encapsulated composite structure in which individual Pt nanoparticles are surrounded by gas-permeable and catalytically active CeO2 shells. Positively charged surfactants on the nanoparticle surfaces are exploited to adsorb negatively charged Ce precursor complexes spontaneously, followed by confined precipitation to form cerium dioxide. This strategy enables the creation of uniformly coated shell structures with tunable thicknesses between 2.9 and 26.5 nm, thereby enabling the investigation of how thickness affects the thermal stability and chemical reactivity of the composite particles. Enhanced metal–support interactions significantly prevent Pt agglomeration, leading to exceptionally high reactivity for methane combustion. With a shell thickness of 13.8 nm, we observe a T10 lower by more than 100 °C with an eight-fold higher reaction rate when compared with a bare mixture of Pt and CeO2 nanoparticles. Furthermore, their cores remain isolated even after heating them to 1000 °C, while complete methane oxidation was maintained for more than 50 hours at 700 °C. These results provide improved guidelines toward the design of a sintering-resistant, high-performance catalyst for use at elevated temperatures.

 Please wait while we load your content...
Please wait while we load your content...