Mild activation of CeO2-supported gold nanoclusters and insight into the catalytic behavior in CO oxidation†
Abstract
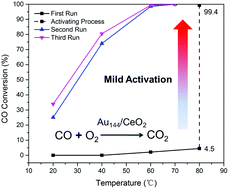
We report a new activation method and insight into the catalytic behavior of a CeO2-supported, atomically precise Au144(SR)60 nanocluster catalyst (where thiolate –SR = –SCH2CH2Ph) for CO oxidation. An important finding is that the activation of the catalyst is closely related to the production of active oxygen species on CeO2, rather than ligand removal of the Au144(SR)60 clusters. A mild O2 pretreatment (at 80 °C) can activate the catalyst, and the addition of reductive gases (CO or H2) can enhance the activation effects of O2 pretreatment via a redox cycle in which CO could reduce the surface of CeO2 to produce oxygen vacancies—which then adsorb and activate O2 to produce more active oxygen species. The CO/O2 pulse experiments confirm that CO is adsorbed on the cluster catalyst even with ligands on, and active oxygen species present on the surface of the pretreated catalyst reacts with CO pulses to generate CO2. The Au144(SR)60/CeO2 exhibits high CO oxidation activity at 80 °C without the removal of thiolate ligands. The surface lattice-oxygen of the support CeO2 possibly participates in the oxidation of CO over the Au144(SR)60/CeO2 catalyst.

 Please wait while we load your content...
Please wait while we load your content...