Highly narrow nanogap-containing Au@Au core–shell SERS nanoparticles: size-dependent Raman enhancement and applications in cancer cell imaging†
Abstract
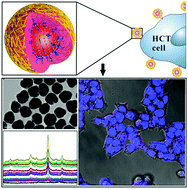
Cellular imaging technologies employing metallic surface-enhanced Raman scattering (SERS) tags have gained much interest toward clinical diagnostics, but they are still suffering from poor controlled distribution of hot spots and reproducibility of SERS signals. Here, we report the fabrication and characterization of high narrow nanogap-containing Au@Au core–shell SERS nanoparticles (GCNPs) for the identification and imaging of proteins overexpressed on the surface of cancer cells. First, plasmonic nanostructures are made of gold nanoparticles (∼15 nm) coated with gold shells, between which a highly narrow and uniform nanogap (∼1.1 nm) is formed owing to polyA anchored on the Au cores. The well controlled distribution of Raman reporter molecules, such as 4,4′-dipyridyl (44DP) and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), are readily encoded in the nanogap and can generate strong, reproducible SERS signals. In addition, we have investigated the size-dependent SERS activity of GCNPs and found that with the same laser wavelength, the Raman enhancement discriminated between particle sizes. The maximum Raman enhancement was achieved at a certain threshold of particle size (∼76 nm). High narrow nanogap-containing Au@Au core–shell SERS tags (GCTs) were prepared via the functionalization of hyaluronic acid (HA) on GCNPs, which recognized the CD44 receptor, a tumor-associated surface biomarker. And it was shown that GCTs have a good targeting ability to tumour cells and promising prospects for multiplex biomarker detection.

 Please wait while we load your content...
Please wait while we load your content...