Computational electronic structure of the bee killer insecticide imidacloprid†
Abstract
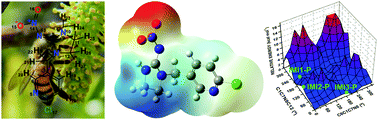
Imidacloprid is the most commonly used neonicotinoid insecticide worldwide. Although it presents no selectivity among insect species, this molecule has been implicated in the deaths of beneficial pollinating insects, such as bees, and in the contamination of water and soil for a long period of time. Knowledge of the structural and electronic features of the imidacloprid agrochemical is still limited; this knowledge is essential for understanding its mechanism of action, especially its interactions with nicotinic acetylcholine receptors (nAChRs), soil and water, and for the development of environmental monitoring techniques and sensors. Thus, a detailed investigation of the chemical structure of imidacloprid was performed, including its protonation state at pH 7.4; the study of one conformer in vacuum and three conformers in the aqueous state below room temperature; a comparison of these conformers with the structure of imidacloprid in the crystalline state and in the estimated imidacloprid acetylcholine receptor binding pocket; its charge distribution; and its HOMO(0) and LUMO(0) orbitals. Improved structural and electronic data were obtained for imidacloprid through state-of-the-art computations performed within the Density Functional Theory (DFT) framework, employing the 6-311++G(d,p) basis set and the M06-2X exchange correlation potential.


 Please wait while we load your content...
Please wait while we load your content...