Amberlite IR-120(H)-mediated “on water” synthesis of novel anticancer ruthenium(ii)–p-cymene 2-pyridinylbenzothiazole (BTZ), 2-pyridinylbenzoxazole (BOZ) & 2-pyridinylbenzimidazole (BIZ) scaffolds†
Abstract
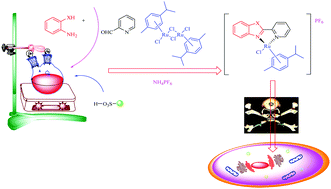
Herein, we have introduced a novel, green and effective methodology for the synthesis of Ru(II)–arene 2-pyridinylbenzothiazole, Ru(II)–arene 2-pyridinylbenzoxazole & Ru(II)–arene 2-pyridinylbenzimidazole complexes in a one-pot sequence. A convenient and clean “on water” synthesis of organoruthenium is reported. Amberlite IR-120(H) has been employed as a reusable catalyst in this reaction. The operational simplicity, excellent yield, ease of product isolation and high chemoselectivity are the main advantages of this method; moreover, this process is economical, safe and environmentally benign. This method offers the opportunity of synthesizing a variety of newer organoruthenium scaffolds for anticancer screening. Most of the compounds presented here have displayed significant cytotoxicity & selectivity against MCF7, Hela and A2780 cell lines compared to cisplatin. Since, [(η6-p-cymene)Ru-2-(6-bromopyridin-2-yl)-benzo[d]thiazole] (4g) was identified as the most potent fluorescent scaffold in terms of cytotoxicity & selectivity in the cancer cell lines, it might be used in cancer theranostics.

 Please wait while we load your content...
Please wait while we load your content...