A water-soluble Pd(ii) complex with a terpyridine ligand: experimental and molecular modeling studies of the interaction with DNA and BSA; and in vitro cytotoxicity investigations against five human cancer cell lines†
Abstract
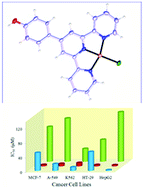
A new Pd(II) complex, [Pd(4-OHPh-tpy)Cl]Cl, with a ligand that is a terpyridine derivative, (4′-(4-hydroxyphenyl)-2,2′,6′,2′′-terpyridine (4-OHPh-tpy)), was prepared and fully characterized. The single crystal structures of 4-OHPh-tpy and its Pd(II) complex were determined by X-ray crystallography. The in vitro studies (UV-Vis spectroscopy, circular dichroism (CD), rheometry, and gel electrophoresis) show that the Pd(II) complex interacts with calf-thymus DNA (CT-DNA) via a combination of covalent, intercalation, and hydrogen bonding interactions, whereas the free 4-OHPh-tpy ligand interacts with DNA mainly through intercalation. The Pd(II) complex cleaves the supercoiled double-stranded DNA under physiological conditions without the need to add an external reductant. The microenvironment and the secondary structure of BSA were also changed in the presence of both the free ligand and the Pd(II) complex. The Pd(II) complex was remarkable in exhibiting cleavage of the BSA at micromolar concentrations and short incubation times at physiological pH and temperature. The anti-cancer effects of the free ligand and the Pd(II) complex were tested in vitro against five human tumor cell lines, including the human breast cancer cell line (MCF-7), lung adenocarcinoma cells (A-549), an erythroleukemic cell line (K562), a colorectal adenocarcinoma cell line (HT-29), and hepatocellular carcinoma (Hep-G2) cells. The Pd(II) complex showed the IC50 values less than those of cisplatin against the MCF-7, A-549, K562, and HT-29 cancer cell lines. Finally, the binding of the Pd(II) complex and 4-OHPh-tpy to BSA and DNA was modeled by molecular docking and molecular dynamic simulation methods.

 Please wait while we load your content...
Please wait while we load your content...